ArtÃculo Original
Argentine Registry of Coronary Angioplasty 2 (RAdAC 2). Early results
Ernesto M Torresani, Arturo Fernández Murga, Alejandro Moguilner, Miguel A Larribau, Juan H Guiroy, Germán L Cafaro, Nicolás A Nitti, Alejandro Cherro, MartÃn F Cisneros Soria, Guillermo R Martino
Revista Argentina de Cardioangiología Intervencionista 2022;(3): 0123-0129 | Doi: 10.30567/RACI/20223/0123-0129
Este artículo no contiene abstract
Los autores declaran no poseer conflictos de intereses.
Fuente de información Colegio Argentino de Cardioangiólogos Intervencionistas. Para solicitudes de reimpresión a Revista Argentina de CardioangiologÃa intervencionista hacer click aquí.
Recibido 2022-04-24 | Aceptado 2022-07-05 | Publicado

Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-SinDerivar 4.0 Internacional.
Introduction
Percutaneous transluminal angioplasty is a therapeutic method that has been established for quite a few years especially for the management of patients with ischemic heart disease. However, the evolution of indications and resources available is remarkable. Not long ago, patients with left main coronary artery obstructions or multivessel disease were only considered eligible for surgery. However, today, “in many cases”, they are often treated through angioplasty. On the other hand, we should also mention the technological advances made with the arrival of new diagnostic tools like fractional flow reserve (FFR), optical coherence tomography (OCT), the evolution of stents (different metal alloys, polymers capable of releasing dugs in a controlled way, etc.), and fully biodegradable platforms. In our setting we conducted the RAdAC trial(1,2) a few years ago (from May 2010 through February 2012) that included 67 medical PCI-capable centers and 3102 patients. This study was conducted to try to recreate it with updated data.
The objective of this manuscript is to presentat the protocol (Appendix 1), and the early in-hospital results.
Material and methods
A protocol was designed (Appendix 1) that was presented and approved by the the Argentine Society of Cardiology Ethics Research Committee. Afterwards, during the first half of 2019, all Argentine PCI-capable centers were invited to participate in this registry (Figure 1). Participants needed to sign a document and commit themselves to filling out the data in a prospective, consecutive, and uninterrupted way, signing the informed consent form, keeping data confidentiality, finishing the follow-up period, and accepting the possibility of monitoring/auditing.
This was a multicenter, prospective, cohort registry. Patients over 21 years of age with ischemic heart disease (whether acute or chronic) treated with percutaneous transluminal angioplasty (PTA) who had given their consent to participate in the registry and had signed the informed consent were included. Only patients incapable of signing the informed consent form and/or whose legal representative refused to sign it were excluded. Patients were included prospective and consecutively for 1 calendar year from the date when the study started. The routine follow-up of each center was conducted and included in the registry after 6 and 12 months. These data could be obtained on-site or through the phone. Each center included 1 lead investigator and 1 associate investigator (Appendix 2) (responsible for following the protocol) and who completed the database through an electronic form.
Statistical analysis
Continuous variables were expressed as mean and standard deviation or median and interquartile range based on their distribution. Categorical variables were expressed as percentages. Event-free survival was assessed using the Kaplan-Meier curves. For the identification of independent event predictors at discharge the multiple logistic regression analysis was used while for the identification of 6 and 12 month-predictors the Cox regression analysis was used. Regarding sub-analyses, continuous variables were compared with parametric or non-parametric methods based on whether they corresponded to Gaussian distribution. Categorical variables, however, were compared using the chi-square test depending on each case. P values < .05 were considered statistically significant.
Results
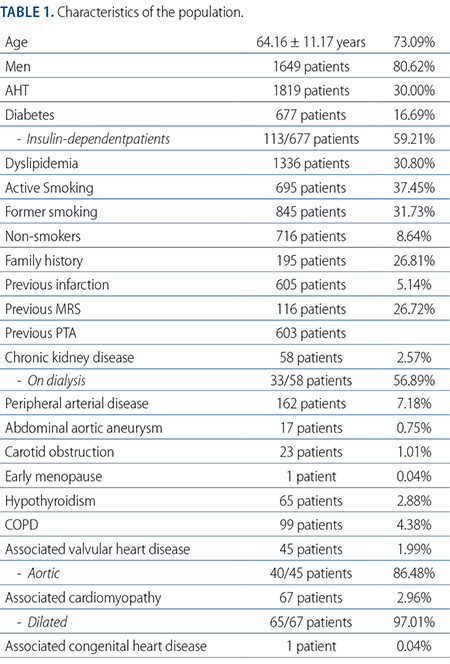
From September 2019 through September 2020, a total of 38 medical centers from 14 different provinces from Argentina, and CABA (Appendix 2) and 2256 patients were studied. Most were men with a mean age of 64 years; 80.62% were hypertensive, 30% were diabetic (16.69% required insulin), 59.21% were dyslipidemic, 68.25% were smokers or former smokers, 26.81% had had a previous infarction, and 31.86% had been treated with a previous revascularization through MRS or angioplasty. A total of 58 patients had chronic kidney disease. A little over half of these patients were on dialysis. Vascular disease was located at a different territory in around 8%. Valvular heart disease was found in 45 patients being 86.48% of aortic origin. Also, cardiomyopathy was found in 67 patients being dilated cardiomyopathy in almost all the cases (Table 1).
Clinical indication was mainly based on unstable clinical signs (Figure 2): acute myocardial infarction (AMI) with or without ST-segment elevation (44.81%), unstable angina (30.46%), and heart failure due to myocardial ischemia (4.05%%). Silent ischemia was present in 5.79% of the cases while stable chronic stable angina pectoris occurred in 14.85% of the cases.
Dominance in coronary circulation was right-sided in 89.24% of the cases, left-sided in 7.97%, and balanced in 2.77%. Severe coronary obstructions were found in the LMCA (5.27%), LAD (59.01%), first diagonal branch (9.92%), LCx (32.91%), lateral branch (13.73%), RCA (41.02%), posterior descending coronary artery (4.51%), and bypass (2.56%) causing 1-vessel (49.62%), 2-vessel (29.49%), and ≥ 3-vessel disease (20.89%).
A total of 2544 PTAs were performed on 2256 patients with an overall primary success rate of 92.93%. The preferred access route was radial (60.3%) followed by the femoral (39.4%), and humeral (0.29%) access routes. Most cases (93.53%) were solved in 1 single procedure while 2 different procedures were needed in 6.26% and 3 in 0.2%. The use of diagnostic methods different from the radiological ones like IVUS, OCT, FFR or iFR (Table 2) was low. However, in ¼ of the patients the use of stent optimization systems like the Stent Boost® was reported.
Devices different from conventional balloons were used for plaque preparation in only 1.77% of the cases (Rotablator®: 0.45% - cutting balloon: 1.32%). Patients were treated with DES (drug eluting-stents) in 93.69%, BMS (bare metal stents) in 5.9%, and DEB (drug eluting-balloons) in 0.4% of the cases. The following were the perioperative complications reported: type 4a AMI (0.53%); type 4b AMI (0.04%); TIA (0.08%); TIMI major bleeding (0.35%) (digestive: 3 patients; femoral puncture: 2 patients; radial puncture: 2 patients; urinary: 1 patient); stent thrombosis (0.35%) (acute and defined in 6 patients, subacute in 2 patients being possible in 1 and probable in another patient). None of the patients had to be referred to undergo MRS during the hospital stay that triggered the PTA that qualified for registry admission. The overall mortality rate was 1.37% being of cardiovascular and non-cardiac causes in 1.24% and 0.13% of the cases.
Discussion
A total of 2544 coronary angioplasties were analyzed in 2256 patients with a high profile of risk. The patients’ mean age was 64.16 ± 11.17 years, 80.62% of whom were hypertensive, 59.21% dyslipidemic, and 30% diabetics. Of these, 16.69% were insulin-dependent and in them clinical instability (unstable angina, AMI with and without ST-segment elevation, and CHF) determined the indication in most of the cases (79.32%). Still, the rate of overall primary success was high as opposed to the rates of complications and mortality that were low.
Another detail we should be paying attention to is that the rate of AMI varied between the first (before April 2020) and the second halves of the study, which amounted to 22% of the cases treated during the first half and 32% of the cases treated during the second, being this difference statistically significant (P < .0001) with the additional detail that within the first half tirofiban was used only in 1 patient compared to 9 during the second half. We should mention that the existing IIb-IIIa inhibitors eptifibatide and abxicimab have not been available in our setting for years. Also that tirofiban was discontinued during the second half of 2019. However, it was reinstated half through 2020. We believe that when primary success was defined (see Appendix 1) including residual obstruction, TIMI grade-3 flow, and myocardial blush 3 too this situation should have had a strong influence.
The rates of dominance and distribution of the obstructions per coronary blood vessel are similar to those already reported. Radial access was the most widely used one (60.3%). However, the femoral access route is still being used in 39.4% of the cases while the humeral one is left for situations of exceptionality only (0.29% of the cases).
The use of plaque preparation devices (Cutting Balloon®, Angiosculp® or Rotablator®) different from conventional balloons and additional diagnostic methods (IVUS, OCT, FFR, and iFR) different from the radiologic ones (used in 3.42% of procedures only) was very low (1.77%). We should mention that when the recruitment phase of this registry occurred, no coronary plaque preparation methods like the ShockWave IVL® or other were available in Argentina.
Radiology imaging systems for stent optimization like StentBoost® or similar were widely used, which is somehow indicative of the renovation of radiology imaging systems in our cath labs today.
Most cases (93.69%) were treated with DES, but very few BMSs (5.9%) and DEBs (0.4%) were used in the coronary territory, which reveals the worldwide tendency on this regard.
There are different considerations that should be made when analyzing the results shown here. For starters, we should say that although PCI-capable centers were invited to participate in Argentine cath labs (nearly 328) via email, WhatsApp, and CACI sessions in FAC and SAC congresses, initially, only 55 centers seemed interested (Figure 1) that eventually signed the participation and commitment form. Of these, only 13 centers included no patients at all, and only 4 started including them irregularly to stop doing so only a few months later actively participating a total of 38 centers (nearly 10%). We can speculate that participating in this kind of registries comes with no benefits (maybe, at best, being quoted in a publications) or that too many requirements were made (need to sign a participation and commitment form including the possibility of authorship) when being included as a participant centers. We should mention that we dealt with centers from 14 different provinces and CABA including 8 out of 38 public hospitals (21%), which creates a heterogeneous sample somehow. Another detail we should take into consideration is that halfway through recruitment (started back in September 2019) the COVID-19 pandemic broke out (somewhere around March 2020). There is no doubt that this totally changed us, reduced the number of patients, and complicated our early intention of auditing the data mined. Therefore, one of the study limitations can be the involuntary sub-registry of certain data (there’s no data on the number of coronary angiographies performed or the number of patients who were eventually excluded). Also, the fact that in 2020 only the most severe patients of all were the ones seeking medical attention.
Conclusions
The characteristics of the population included in the RAdAC 2 registry are a sample of the type of patients often treated at the centers where it was conducted being “probably” a reflection of what goes on in general. Clinical instability (unstable angina, AMI with/ST-segment elevation, AMI without/ST-segment elevation, and CHF) determined the indication in most cases. The rates of coronary dominance and distribution of the obstructions per coronary blood vessel are indicative of the rates usually known. High rates of overall primary success and low rates of complications and mortality were reported. Plaque preparation devices different from conventional balloons and additional diagnostic methods different from radiology imaging systems (IVUS, OCT, FFR, and iFR) weren’t used that much. However, radiology imaging systems for stent optimization were very much used. Most cases (93.69%) were treated with DES. Radial access was the most commonly used of all.
APPENDIX 1
Argentine Registry of Coronary Angioplasty 2 (RAdAC 2)
http://caci.org.ar/assets/uploads/protocolo-radac2
Endpoints
Primary endpoints: To know the rate of primary success, the characteristics of the target population, the modality of revascularization, and the incidence rate of in-hospital events.
Secondary endpoints: To know the incidence rate of out-of-hospital events at the 6-and-12-month follow-up after the hospital discharge that triggered the early intervention.
Material and methods
The data included were age, sex, weight, height, risk factors (AHT, insulin dependent or not diabetes, smoking status, family history, and/or dyslipidemia), previous infarction, previous MRS, previous PTA, heart failure (CHF), stroke, kidney disease (including creatinine levels and whether the patient is on dialysis or not), peripheral vascular disease, abdominal aortic aneurysm, carotid artery disease, early menopause, hypothyroidism, chronic obstructive pulmonary disease (COPD), valvular heart disease, cardiomyopathy, and/or congenital heart disease (whether corrected or not).
The clinical indication was based on the presence of chronic stable angina, silent ischemia, positive functional test, heart failure, arrhythmia, AMI with ST-segment elevation [characterized by: a) Signs of ischemia (angina pectoris or equivalent) for, at least, 20 minutes; b) Acute ST-segment elevation, over 1 mm, in 2 adjacent leads or c) New or presumably new-onset LBBB (left bundle branch block), AMI without ST-segment elevation [patients in whom the ECG does not show any ST-segment elevation with higher biomarkers (troponin and/or CPK-MB), and presence of any of the following clinical signs: a) Resting angina (angina starts while the patient is at rest); b) Early-onset angina (< 2 months); c) Crescendo angina (angina of an increased intensity, duration and/or frequency)], unstable angina or resuscitated cardiac arrest. In cases of AMI with ST-segment elevation the strategy used was direct PTA—whether pharmacoinvasive or bailout—and reperfusion—whether successful or not—plus the times involved (symptom-to-balloon, and door-to-balloon), and Killip-Kimball classification.
The indication for PTA was considered as: a) Scheduled: Patients who required elective PTA but in whom the PTA could be delayed without further risks involved. b) Urgent: Patients who require to undergo the procedure during the hospital stay for medical reasons unable to be discharged without the procedure been performed. c) Emergency: Patients not scheduled to be operated on with ongoing refractory cardiac compromise. The procedure cannot be delayed regardless of the time of the day.
Left ventricular systolic function was categorized as not assessed or normal (ejection fraction [EF] ≥ 55%), mild (EF, 46% to 55%), moderate (EF, 45% to 36%) or severe compromise (EF, ≤ 35%) depending on whether it was assessed prior to the angioplasty that triggered the admission to the registry through echocardiography, radioisotopic ventriculography, ventriculography through cardiac catheterization or cardiac angioresonance.
Coronary angiography was categorized as: a) dominance; b) severe obstructions (≥ 70%) in main vessels (left anterior descending, left circumflex or right coronary arteries); c) severe obstructions (≥ 70%) in branches (posterior descending, first diagonal or latero-ventricular coronary arteries) with diameters ≥ 2.0 mm; d) severe left main coronary artery obstructions (≥ 50%); e) severe obstructions in bridges (mammary, radial, venous) (≥ 70%). The number of blood vessels with severe obstructions and on treatment were quantified too.
Procedures were performed following the protocols of each center, and they were left to the operator’s criterion. The most widely used strategies in the registry a) access route (whether radial, humeral or femoral); b) direct stenting or plaque preparation (balloon, Cutting Balloon®, Angiosculp®, Rotablator®, etc.); c) type of stent (BMS, DES, brand); d) thrombus aspiration (yes or no); e) balloon pump (yes or no); f) use of intracoronary diagnostic imaging modalities (IVUS, OCT, FFR and/o iFR); g) use of radiology imaging systems to optimize stent visualization (Stent Boost®, Stent Clear®, Stent Viz®, etc.).
The pharmacological therapies used were a) anticoagulants (sodium heparin or bivalirudin); b) fibrinolytic drugs (rTPA or streptokinase); c) IIb-IIIa inhibitors (tirofiban), and d) antiplatelet therapy drugs (aspirin, clopidogrel, prasugrel and/o ticagrelor).
The characteristics of the obstructions were categorized based on the definitions provided by the Syntax Score(3-4) like a) ostial; b) reestenosis; c) severe tortuosity; d) occlusion (in the presence of collateral circulation the Rentrop classification would be used(5)); e) bifurcation (in this case using the Medina classification(6)); f) thrombus (also using the TIMI Thrombus Score(7)); g) calcification; h) length > 20 mm; i) diffuse disease.
Both the baseline and final TIMI flow grade(8) and myocardial blush(9) were included. Successful angioplasty was considered as residual obstructions < 30% with TIMI flow grade-3, and myocardial blush 3.
Complications or procedural events were categorized as A) none; B) vascular access related [a) radial: spasm, hematoma (Bertrand classification(10)), dissection, perforation, pseudoaneurysm, hand ischemia, AV fistula, endothelial eversion, granulomatose inflammation, infection, need for surgery; b) femoral: large hematoma (< 10 cm), pseudoaneurysm, AV fistula, limb ischemia (due to dissection or thrombosis), infection, need for surgery; C) coronary branch occlusion; D) no reflow phenomenon or slow flow; E) coronary perforation; F) cardiac tamponade; G) aortic dissection; H) adverse reaction to the contrast agent; I) AV block requiring intraoperative pacemaker implantation; J) need for resuscitation/cardioversion.
The in-hospital events reported were A) perioperative AMI: occurred within the 48 hours following the index procedure; a) AMI post-PTA (type 4a): High and low biomarker levels (troponina or CK-MB) 3 times above the normal reference value (percentile 99 of the upper reference limit) in patients in whom normal baseline values are assumed within a certain degree of normalcy. In the presence of previous CKD, CHF or other abnormalities that can damage the patient, high or low reference values will be assessed in association with such baseline normal values; b) AMI post-PTA (type 4b): AMI associated with stent thrombosis whenever angiographically documented or through autopsy; B) reinfarction: when biomarker values remain stable or drop, they go up 20% in a second sample within the next 3 to 6 hours; C) spontaneous AMI: occurred within the next 48 hours following the procedure. Increased or reduced cardiac biomarker levels, preferably troponin, with, at least, 1 of the registries above the reference value (> percentile 99th of the upper reference limit) for normal individuals. One or more of the following clinical signs of myocardial ischemia can occur: 1) ischemic symptoms, 2) changes to the ECG suggestive of new-onset ischemia (ST-T alterations, loss of voltage to the R wave, new left bundle branch block); 3) Development of Q waves in 2 or more adjacent leads on the ECG; 4) Images showing new loss of viable myocardium or regional motility abnormalities; D) sudden death; E) need for target vessel revascularization (through PTA or MRS); F) TIMI bleeding(11) (major or minor); G) Ischemic or hemorrhagic stroke (major or minor); H) death (whether cardiovascular or not); I) stent thrombosis based on the classification established by the Academic Research Consortium.(12) Patients were followed for a full year and, if necessary, antiplatelet therapy was discontinued. The reason for such discontinuation would have to be given. Events considered relevant enough for such discontinuation were death, acute myocardial infarction, need for new target vessel revascularization (unscheduled) (MRS or PTA) or stroke (ischemic or hemorrhagic).
APPENDIX 2
Participant centers and investigators – RadAC 2
Buenos aires
El Palomar: Hospital Nacional Alejandro Posadas, Dr. Villegas, Dr. Miguel O., and Dr. Riolo, Federico M.; Quilmes: Sanatorio Modelo Quilmes, Dr. Torresani, Ernesto M., and Dr. Martino, Guillermo R.; Olavarría: Hospital Héctor Cura, Dr. Violante, Dr. Ricardo M., and Dr. Tancredi, Valentina E.; La Plata: Hospital Español de La Plata, Dr. Grinfeld, Diego, and Dr. Fuertes, Fernando; Instituto Médico Platense; Dr. Nitti, Nicolás A., and Dr. Guridi, Cristian; Pergamino: Hospital San José, Dr. Bahamonde, Dr. Luis A., and Dr. Sucarilipa, Edgar; Mar del Plata: Sanatorio Belgrano, Dr. Delacasa, Arturo A., Dr. Fernández Trivino, and Dr. Marcos A.; Clínica 25 demayo, Dr. Iravedra, Dr. Jorge A. M., and Dr. Bruno, Rodrigo; Temperley: Sanatorio Juncal, Dr. Gadda, Carlos E., and Dr. Civitarese, Andrés.
CABA
Diagnóstico Mediter-Sanatorio Dr. Julio Méndez, Dr. Cafaro, Germán L., and Dr. Florencio, Anahí D.E.; Clínica Bazterrica, Dr. Leguizamón, Dr. Jorge H., and Dr. Carrera, Juan P.; Sanatorio San José, Dr. Leguizamón, Dr. Jorge H., and Dr. Escalante, José M.; Clínica Santa Isabel, Dr. Fernández, Alejandro A., and Dr. Pazos, Cristian; Sanatorio Franchin, Dr. Chambre, Dionisio, and Dr. Moguilner, Alejandro; Hospital Ramos Mejía, Dr. Stürmer, Cristiano, and Dr. Moguilner, Alejandro; Clínica Adventista de Belgrano, Dr. Cherro, Alejandro, and Dr. Videla Lynch, Angeles; Sanatorio Trinidad Palermo, Dr. Palacios, Alejandro, and Dr. Fernández, Juan J.; Sanatorio Mater Dei, Dr. Palacios, Alejandro, and Dr. Fernández, Juan J.
Chaco
Resistencia: Instituto Cardiovascular del Chaco, Dr. Guiroy, Juan C., Dr. Wirz, Fabrizio, and Dr. Niello, Esteban N.
Chubut
Comodoro Rivadavia: Clínica del Valle, Dr. Manos, Dr. Eustaquio J., and Dr. Piasentini, J.
Córdoba
Córdoba Capital: Hospital Privado Universitario de Córdoba, Dr. Ballarino, Dr. Miguel A., and Dr. Amuchástegui, Marcos; Clínica Vélez Sarsfield, Dr. Rubio, Mariano C., and Dr. Mercado, Natalia L.; Sanatorio Francés, Dr. Cisneros Soria, Dr. Martín F., and Dr. Trejo, Santiago L.
Formosa
Formosa Capital: Hospital de Alta Complejidad Pte. Juan Domingo Perón, Dr. Vega, Alejandra S., and Dr. Acosta, Marisa.
La Rioja
La Rioja Capital: Instituto de Medicina Endovascular IMEV SRL, Dr. Ballarino, Dr. Daniel E., and Dr. Vázquez, Roberto R.
Mendoza
Guaymallén: Hospital Santa Isabel de Hungría, Dr. Larribau, Dr. Miguel A., and Dr. Irusta, G.; Mendoza Capital: Clínica de Cuyo S.A., Dr. Larribau, Dr. Miguel A., and Dr. Guzzanti, Diego A., Mendoza Capital: Hospital Español de Mendoza, Dr. Larribau, Dr. Miguel A., and Dr. D’Amico, Guido.
Misiones
Posadas: IOT Sanatorio Integral, Dr. Estevez, Santiago M. - Dr. Román, Raúl A., and Dr. Romano, José R.; Hospital Escuela de Agudos Dr. Ramón Madariaga, Dr. Duarte, Dr. Ernesto R. – Dr. Babi, Carlos A., and Dr. Moreschi Aquino, Enzo.
Salta
Salta Capital: Hospital San Bernardo, Dr. Farah, Dr. Miguel A. - Dr. Pereira, Juan M. – Dr. Pérez Solivellar, Pablo, and Dr. Paez, Rosa.
San Luis
Villa Mercedes: Instituto Cardiovascular de Mercedes, Dr. Bravo, Alfredo.
Santa Cruz
Río Gallegos: Hospital Regional de Río Gallegos, Dr. Biagioni, Corina, and Dr. Mehenhuech, Pablo.
Santa Fe
Santa Fe Capital: Sanatorio Mayo S.A., Dr. Licheri, Alberto J., and Dr. Licheri, Marisa C; Sanatorio Diagnóstico Santa Fe, Dr. Licheri, Alberto J., and Dr. Quarchioni, Esteban); Rosario: Sanatorio Británico de Rosario, Dr. Zanuttini, Daniel A., and Dr. Cúneo, Tomás.
Tierra del Fuego
Río Grande: Clínica CEMEP, Dr. Mollon, Dr. Ana P., and Dr. Paganini, Ignacio L.
Tucumán
Tucumán Capital: Instituto de Cardiología SRL San Miguel de Tucumán, Dr. Fernández Murga, Arturo, and Dr. Cruzado, José.
-
Cherro A, Fernández Pereira C, Torresani EM y cols., en representación de los Investigadores del RAdAC. Resultados hospitalarios y factores asociados con morbimortalidad del Registro Argentino de Angioplastia Coronaria (RAdAC). Rev Arg Cardiol 2012; 80:452-460.
-
Fernández Pereira C, Scuteri A, Allín J y cols., en representación de los Investigadores del RAdAC. Resultados intrahospitalarios de los pacientes con enfermedad coronaria tratados con angioplastia en el país. Registro Argentino de Angioplastia Coronaria (RAdAC). Rev Arg Cardioangiol Interven 2013;(01):49-58.
-
Sianos G, Morel M-A, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroInterv 2005;1:219-227.
-
http://syntaxscore.org/calculator/start.htm
-
Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985 Mar;5(3):587-92.
-
Medina A, Suárez de Lezo J, Pan M. Una clasificación simple de las lesiones coronarias en bifurcación. Rev Esp Cardiol. 2006; 59:183.
-
Gibson CM, de Lemos JA, Murphy SA, et al. Combination therapy with abciximab reduces angiograhically evident thrombus in acute myocardial infarction – a TIMI 14 substudy. Circulation 2001;103,2550-4.
-
The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med 1985;312(14):932-6.
-
van ‘t Hof AWJ, Liem A, Suryapranata H, et al. Angiographic Assessment of Myocardial Reperfusion in Patients Treated With Primary Angioplasty for Acute Myocardial Infarction Myocardial Blush Grade. Circulation 1998;97(23):2302-6.
-
Bertrand OF. Acute forearm muscle swelling post transradial catheterization and compartment syndrome: prevention is better than treatment! Catheter Cardiovasc Interv 2010;75(3):366-8.
-
Mehran R, Rao SV, Bhatt DL, et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials. A Consensus Report From the Bleeding Academic Research Consortium. Circulation 2011;123:2736-47.
-
Cutlip DE, Windecker S, Mehran R, on behalf of the Academic Research Consortium. Clinical End Points in Coronary Stent Trials A Case for Standardized Definitions. Circulation 2007;115:2344-51.
-
Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Col Cardiol 2018;72:2231-64.
Ernesto M Torresani
Researcher of RAdAC 2. (ORCID: 0000-0002-6543-1966).
Arturo Fernández Murga
Researcher of RAdAC 2.
Alejandro Moguilner
Researcher of RAdAC 2.
Miguel A Larribau
Researcher of RAdAC 2.
Juan H Guiroy
Researcher of RAdAC 2.
Germán L Cafaro
Researcher of RAdAC 2.
Nicolás A Nitti
Researcher of RAdAC 2.
Alejandro Cherro
Researcher of RAdAC 2.
MartÃn F Cisneros Soria
Researcher of RAdAC 2.
Guillermo R Martino
Researcher of RAdAC 2, on behalf of the RAdAC 2 researchers.
Autor correspondencia
Ernesto M Torresani
Researcher of RAdAC 2. (ORCID: 0000-0002-6543-1966).
Correo electrónico: etorresani@intramed.net
Para descargar el PDF del artículo
Argentine Registry of Coronary Angioplasty 2 (RAdAC 2). Early results
![]() Haga click aquí
Haga click aquí
Para descargar el PDF de la revista completa
Revista Argentina de CardioangiologÃa intervencionista, Volumen Año 2022 3
Revista Argentina de CardioangiologÃa intervencionista
Issue # 3 | Volumen
12 | Año 2022
What is more important to have favo...
Alfredo E RodrÃguez
Hypoalbuminemia: a biological expre...
Carlos Fernández-Pereira
Clinical impact of hypoalbuminemia ...
Cristian Maximiliano Garmendia y cols.
Argentine Registry of Coronary Angi...
Ernesto M Torresani y cols.
Early experience in Argentina and L...
Carlos Giuliani y cols.
Follow-up of a patient with transca...
Alejandro Ãlvarez Iorio y cols.
Left atrial appendage occlusion wit...
Pablo D Liva y cols.
Health legislation in Argentina
Alejandro Octavio Palacios
The proud feeling of belonging
MartÃn Cisneros
Argentine Registry of Coronary Angioplasty 2 (RAdAC 2). Early results
Autores
Ernesto M Torresani, Arturo Fernández Murga, Alejandro Moguilner, Miguel A Larribau, Juan H Guiroy, Germán L Cafaro, Nicolás A Nitti, Alejandro Cherro, MartÃn F Cisneros Soria, Guillermo R Martino
Publicación
Revista Argentina de CardioangiologÃa intervencionista
Editor
Colegio Argentino de Cardioangiólogos Intervencionistas
Fecha de publicación
2022-09-30
Registro de propiedad intelectual
© Colegio Argentino de Cardioangiólogos Intervencionistas